Preprint『Construction of Shape-Persistent All-sp2 Square Nanohoops via the Formation of Multiple Imine Bonds』がChemRxivにて公開されました
Construction of Shape-Persistent All-sp2 Square Nanohoops via the Formation of Multiple Imine Bonds
Takashi Harimoto,* Yasutomo Segawa*
ChemRxiv DOI: 10.26434/chemrxiv.10001993/v1
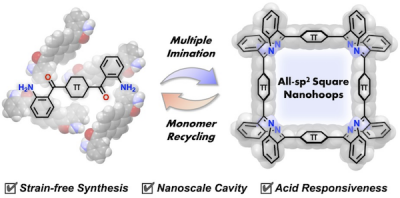
Shape-persistent all-sp2 square nanohoops represent a synthetic challenge due to the difficulties associated with maintaining bond angles of ca. 90° between adjacent π-conjugated panels. Here, we report the rational design and synthesis of square-shaped nanohoops by employing dibenzo[b,f][1,5]diazocine as rigid perpendicular units. Density-functional-theory (DFT) calculations suggested that the formation of cyclic tetramers is thermodynamically favored. Accordingly, the acid-mediated formation of multiple imine bonds via condensation of monomers bearing 2-aminobenzoyl groups afforded cyclic tetramers in yields of up to 60%. Single-crystal X-ray diffraction analyses confirmed square geometries with preserved panel planarity and tunable internal cavities. The strategy is applicable to a variety of panels (benzene, biphenyl, terphenyl, and pyrene), whereby panel rigidity controls tetramer selectivity. The thus obtained square nanohoops exhibit reversible acid responsiveness and enable high-yield monomer recovery via acid hydrolysis—a unique recycling strategy currently unattainable to conventional cross-coupling methods. This study hence establishes a versatile platform for the construction of shape-controlled π-conjugated macrocycles with tunable properties.



