論文『ディスオーダーした有機半導体ヘリンボーン層の電子回折構造解析』がChem.Mater.に掲載されました
Emerging Disordered Layered-Herringbone Phase in Organic Semiconductors Unveiled by Electron Crystallography
Satoru Inoue,* Kiyoshi Nikaido, Toshiki Higashino, Shunto Arai, Mutsuo Tanaka, Reiji Kumai, Seiji Tsuzuki, Sachio Horiuchi, Haruki Sugiyama, Yasutomo Segawa, Kiyofumi Takaba, Saori Maki-Yonekura, Koji Yonekura, Tatsuo Hasegawa*
Chem. Mater. 2021, ASAP. DOI: 10.1021/acs.chemmater.1c02793
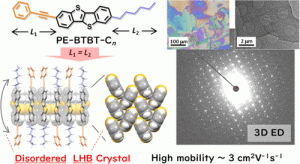
π拡張有機分子の二次元層状結晶相および液晶相の制御は、有機電子材料およびデバイスの可能性を拡張するために重要です。この研究では、[1]ベンゾチエノ[3,2-b] [1]ベンゾチオフェン(BTBT)を2つの異なる置換基、すなわちフェニルエチニル(PE)とアルキルとで非対称化することに基づく独自の溶液処理可能な有機半導体を開発しました。スメクチック液晶相に類似した特徴的な層状液晶相は、置換基の長さがほぼ同じであるn = 6のPE-BTBT-Cnで得られました。 BTBT部分は、剛体の層状ヘリンボーン(LHB)パッキングを維持しますが、分子の長軸は完全な配向の乱れを示しました。このパッキングをdisordered LHB(d-LHB)と名付けました。その独特の形状は、微結晶電子線回折結晶学の新しい技術によって分析できます。分子間コア間相互作用はd-LHBパッキングを安定させ、約3 cm2 V–1 s–1の比較的高い電界効果移動度を可能にしました。対照的に、より長いアルキル鎖を持つPE-BTBT-Cn(n = 8、10、12)は、二重層タイプのLHB(b-LHB)を構成することにより、約7 cm2 V–1 s–1の高い移動度を示しました。
The control of two-dimensional layered crystalline and/or liquid crystalline phases for π-extended organic molecules is crucial for expanding the potential of organic electronic materials and devices. In this work, we develop unique solution-processable organic semiconductors based on the unsymmetric substitution of [1]benzothieno[3,2-b][1]benzothiophene (BTBT) with two different substituents, namely, phenylethynyl (PE) and normal alkyl with different chain lengths n (−CnH2n+1), both of which exhibit structural flexibility while maintaining the rod-like nature over the entire molecule. A distinctive layered solid crystalline phase, analogous to the smectic liquid crystalline phase, is obtainable in PE-BTBT-Cn at n = 6, where the substituent lengths are almost the same. The BTBT moiety maintains a rigid layered-herringbone (LHB) packing, whereas the molecular long axis exhibits a complete orientational disorder. We refer to this packing as disordered LHB (d-LHB), the unique geometry of which can be analyzed by the emerging technique of microcrystal electron diffraction crystallography. The intermolecular core–core interactions stabilize the d-LHB packing, enabling a relatively high field-effect mobility of approximately 3 cm2 V–1 s–1. In contrast, PE-BTBT-Cn with longer alkyl chains (n = 8, 10, 12) exhibits higher mobility of approximately 7 cm2 V–1 s–1 by constituting bilayer-type LHB (b-LHB), which is associated with the unsymmetrical length of the substituents. We discuss the correlation and competition among the d-LHB, b-LHB, and smectic liquid crystalline phases based on the structural, thermal, and transistor characteristics. These findings demonstrate the controllability of various phases in layered organic semiconductors.



