Publication: Synthesis of penta- and hexa(3,4-thienylene): Size-dependent structural properties of cyclic oligothiophenes
Synthesis of penta- and hexa(3,4-thienylene): Size-dependent structural properties of cyclic oligothiophenes
Mai Nagase, Sachiko Nakano, Yasutomo Segawa*
Chem. Commun. 2023, Accepted Manuscript. DOI: 10.1039/D3CC03508E (Gold Open Access)
ChemRxiv DOI: 10.26434/chemrxiv-2023-wc8sl
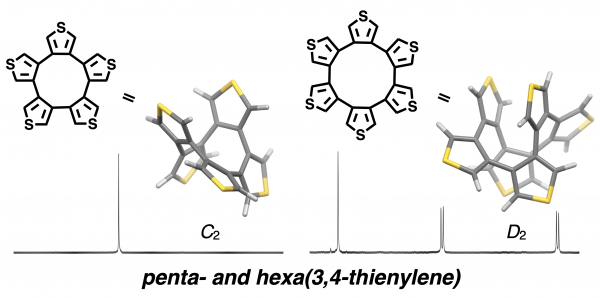
Macrocyclic polyaromatic molecules are interesting materials that exhibit a wide variety of electronic and optical properties derived from their structures, but they are often synthetically challenging because of the ring strain associated with their macrocyclic structures. In this study, we have succeeded in synthesizing 3,4-pentathienylene (5T) and 3,4-hexathienylene (6T), in which all five and six thiophenes are linked at the 3,4-positions (β-positions), using Ni-catalyzed borylation, Pd-catalyzed cross-coupling and Ni-mediated homocoupling reactions. X-ray crystallographic analysis confirmed the C2 and D2 symmetries of 5T and 6T, respectively. Interestingly, the 1H NMR spectra of the two molecules were very different: 5T had a single singlet, whereas 3 different signals were observed for 6T, and these remained unchanged at both low and high temperatures. The isomerization barriers of 5T and 6T calculated by DFT method were 5.0 kcal/mol and 26.5 kcal/mol, respectively, and the difference in isomerization rate was the reason for the difference in NMR spectra. The synthesized 5T and 6T are useful as a platform for the synthesis of novel polycyclic π-conjugated compounds utilizing the macrocyclic nonplanar structures and the active α-positions.



